Robotic transabdominal preperitoneal mesh repair of an inguinal hernia using the Hugo™ Robotic Assisted Surgery system—a case report of the first Australian clinical experience in general surgery
Highlight box
Key findings
• It is feasible to use the Hugo™ Robotic Assisted Surgery (RAS) system to perform transabdominal preperitoneal mesh repair of an inguinal hernia.
What is known and what is new?
• Minimally invasive platforms have been shown to be safe and effective in the repair of inguinal hernias.
• Here we have demonstrated the feasibility of the Hugo™ RAS system in inguinal hernia repair.
What is the implication, and what should change now?
• Further studies are required to demonstrate equivalence between the Hugo™ RAS system and existing minimally invasive platforms in inguinal hernia repair.
Introduction
Robotic surgery has increased in utility in general surgical subspecialties over the last few decades, and the da Vinci system (Intuitive Surgical, Sunnyvale, CA, USA) has been the main platform in use (1-3). But the industry is expanding. In Australia, the Versius® surgical system (CMR Surgical, Cambridge, UK) and the Hugo™ Robotic Assisted Surgery (RAS) system (Medtronic, Minneapolis, MN, USA) have become available for clinical use in general surgery. Both these platforms have moved away from a single patient cart carrying all four robotic arms to a modular design, in which separate patient carts each carry a single robotic arm. Unlike the DaVinci system, Hugo™ RAS and Versius® feature an open surgeon console and have abandoned the pincer grip hand controller. The Hugo™ RAS system has been used in limited clinical settings worldwide. Australia is amongst the first to approve the use of Hugo™ RAS in general surgery with Therapeutic Goods Administration (TGA) approval in late 2022. We performed the first general surgical procedures using Hugo™ RAS in Australia. From this early experience, we present a case in accordance with the CARE reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-23-56/rc).
Case presentation
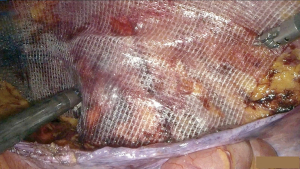
We present our approach to an inguinal hernia repair using the system and demonstrate its feasibility in the accompanying video vignette (Video 1). The patient is a 74-year-old man with a symptomatic reducible right indirect inguinal hernia. He underwent a transabdominal preperitoneal (TAPP) mesh repair (Figure 1) using Hugo™ RAS and was discharged uneventfully the following morning. At 4-week follow-up, there was no evidence of recurrence, or other complications.
All procedures performed in this study were in accordance with the ethical standards of our institution and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The main difference between the Hugo™ RAS and the da Vinci Xi lies in the modularity of arms. From a surgeon’s perspective, this has impacts on theatre set-up and docking, necessitating interdisciplinary team-based training. Akin to the older Da Vinci Si platform, a carefully considered approach to cart positioning and port placement is needed to minimise clashing and optimise access, efficiency, and patient safety. Other differences, such as the open console and “easy grip” control system, have minor impacts on procedural flow. The burden of navigating the initial experience with a new robotic platform is shouldered by early adopters who need to ensure optimal patient safety and outcomes.
Conclusions
Here we share our experience with the Hugo™ RAS for TAPP mesh repair of an inguinal hernia. Further studies are required to demonstrate equivalence between the Hugo™ RAS system and existing minimally invasive platforms in inguinal hernia repair.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-23-56/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-56/coif). K.C.P.T. reports receiving paid lectureship, proctorship, and educational grants for fellow-training from Device Technologies Australia; and receiving paid lectureship and proctorship from Medtronic Australasia and DTG Medical Singapore. F.M.B. reports receiving grants, consulting fee and paid lectureship and proctorship from Medtronic Global, Intuitive Surgical, Asensus Surgical and Virtual Incision. He is an advisory board member of Medtronic Global and receives equipment from Asensus Surgical. A.K. reports receiving paid lectureship and proctorship from Medtronic Australasia. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of our institution and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leal Ghezzi T, Campos Corleta O. 30 Years of Robotic Surgery. World J Surg 2016;40:2550-7. [Crossref] [PubMed]
- Peters BS, Armijo PR, Krause C, et al. Review of emerging surgical robotic technology. Surg Endosc 2018;32:1636-55. [Crossref] [PubMed]
- Cepolina F, Razzoli RP. An introductory review of robotically assisted surgical systems. Int J Med Robot 2022;18:e2409. [Crossref] [PubMed]
Cite this article as: Phan-Thien KC, Bianco FM, Khalessi A. Robotic transabdominal preperitoneal mesh repair of an inguinal hernia using the Hugo™ Robotic Assisted Surgery system—a case report of the first Australian clinical experience in general surgery. Ann Laparosc Endosc Surg 2024;9:17.