
Endoscopic resection of non-papillary neoplastic lesions of the duodenum: a narrative review of clinical application and techniques
Introduction
Background
Non-ampullary duodenal polyps are encountered incidentally in approximately 0.5–5% of routine esophagogastroduodenoscopies (EGD). Recent studies suggest the incidence of these lesions is increasing, which may be multifactorial and due to environmental factors and/or technological advancements that have improved lesion identification (high definition gastroscopes, virtual chromoendoscopy, clear distal attachment devices, high-resolution side-viewing duodenoscopes) (1-3).
Rationale
Although most duodenal lesions are asymptomatic, some can be associated with bleeding (overt or obscure), anemia, obstruction, dyspepsia, abdominal pain, or intussusception (2,4,5). Additionally, some duodenal lesions are neoplasms with underlying malignancy potential. Endoscopic resection is generally more favorable than surgical resection, whenever feasible, for premalignant duodenal neoplasia (1,2,6). Prior to attempting endoscopic resection of duodenal lesions, it is critical for the endoscopist to understand the nuances of duodenal anatomy as well as the various resection techniques and their impact on the efficacy and safety of the procedure.
Objective
In this narrative review, we aim to highlight the fundamental aspects of duodenal anatomy that make endoscopic resection in this area so challenging and to summarize the current literature regarding the technical aspects, efficacy, and safety of various techniques and approaches for endoscopic resection of duodenal lesions. We present this article in accordance with the Narrative Review reporting checklist (available at https://ales.amegroups.com/article/view/10.21037/ales-23-34/rc).
Methods
A literature review was performed using PubMed to search for studies and articles published through to September 30, 2023. The terms used in the search included: “duodenal polyp”, “duodenal adenoma”, “duodenal polyp resection”, “endoscopic duodenal resection”, “duodenal endoscopic mucosal resection”, “non-ampullary duodenal polyps”, “duodenal endoscopic submucosal dissection”, “cold snare polypectomy of duodenum”, “duodenal hamartoma”, “duodenal subepithelial lesion”, “duodenal brunner gland hyperplasia”, “duodenal ectopic gastric tissue”, “duodenal gastrointestinal stromal tumor”, “duodenal lipoma”, “duodenal neuroendocrine tumor”, “duodenal carcinoid”. Table 1 presents detailed search strategy.
Table 1
| Items | Specification |
|---|---|
| Date of search | June 1, 2023 to September 30, 2023 |
| Database searched | PubMed |
| Search terms used | “duodenal polyp”, “duodenal adenoma”, “duodenal polyp resection”, “endoscopic duodenal resection”, “duodenal endoscopic mucosal resection”, “non-ampullary duodenal polyps”, “duodenal endoscopic submucosal dissection”, “cold snare polypectomy of duodenum”, “duodenal hamartoma”, “duodenal subepithelial lesion”, “duodenal brunner gland hyperplasia”, “duodenal ectopic gastric tissue”, “duodenal gastrointestinal stromal tumor”, “duodenal lipoma”, “duodenal neuroendocrine tumor”, “duodenal carcinoid” |
| Timeframe | 1995–2023 |
| Inclusion criteria | Focus was placed on reviews and original studies written in the English language about duodenal lesions and endoscopic resection of these lesions |
| Selection process | Search conducted by R.C.D.B. |
Data review
While there are many similarities to colorectal endoscopic resection, the duodenum has multiple unique characteristics which are critical to understand prior to considering endoscopic resection in this location. Compared to the colon, the wall of the duodenum is thinner and more vascular which increases the risk of perforation and bleeding. The fixed position of the duodenum and the narrower lumen limit maneuverability of the endoscope and make optimal positioning for resection more challenging. Additionally, the location of the lesion in relation to the minor and major papilla must be considered, as papillary involvement greatly alters the procedural approach and risk (2,7,8). Lastly, a complication like duodenal perforation may necessitate a more extensive surgical intervention, such as a pancreatoduodenectomy (Whipple) operation in some cases (9).
Types of duodenal lesions
Prior to attempting any endoscopic tissue removal in the duodenum, it is critical for the endoscopist to have a good understanding of the type of lesion being resected.
Mucosal-based lesions
Adenomas
Adenomas are the most common type of duodenal polyp. These lesions have the potential for transforming from adenoma to adenocarcinoma in two different ways. The intestinal-type lesions (which represent approximately 90% of duodenal adenomas) follow an adenoma-to-carcinoma pathway similar to colonic lesions, whereas gastric-type lesions (most often found in the duodenal bulb) can transition to malignancy through gastric duodenal metaplasia and are associated with a worse prognosis (1,2,10,11). Duodenal adenomas have higher rates of villous histology than colorectal adenomas, so it is thought their risk of malignant transformation is higher compared to a similar-sized lesion in the colorectum (12). Therefore, endoscopic resection should be considered for all duodenal adenomas (1,8). Lesions thought to be particularly high-risk for having advanced histology include size >1 cm, depressed or ulcerated portion of the lesion (Paris 0–IIc), irregular surface pattern, type V Kudo pit pattern, and failure to lift with attempted submucosal injection (8). Duodenal adenomas may appear as a localized patch of milky-white or red-colored mucosa anywhere in the duodenum, range from small to large and have almost any morphology within the Paris classification system, though flat or sessile lesions are the most common (Figures 1-6) (1,2,13). Adenomas can involve the ampulla, so assessment and documentation of the lesion in relation to the major and minor papilla are critical (6). Chromoendoscopy [including virtual chromoendoscopy with narrow-band imaging (NBI), as well as dye-based chromoendoscopy using methylene blue or indigo carmine] may increase duodenal adenoma detection rate, and image-enhanced endoscopy (by using technology such as NBI or dye-based chromoendoscopy by using methylene blue or indigo carmine) has been shown to be helpful in distinguishing neoplastic and non-neoplastic lesions as well as low-grade and high-grade dysplasia and adenocarcinoma (1,14,15).

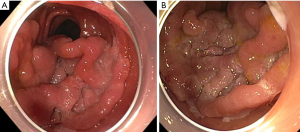



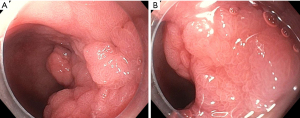
Non-ampullary duodenal adenomas may arise sporadically or as a part of an underlying familial syndrome, particularly familial adenomatous polyposis (FAP) or MUTYH-associated polyposis. Approximately 40% of these lesions are sporadic non-ampullary duodenal adenomas (SNADAs) which tend to arise more commonly as a solitary lesion in the second portion of the duodenum with a higher prevalence in older patients (at 60–80 years of life) (2,4). Independent risk factors for having SNADAs include Barrett’s esophagus [odds ratio (OR) 4.23, 95% confidence interval (CI): 2.17–8.25], current tobacco smoking (OR 3.35, 95% CI: 1.79–6.30), and the presence of fundic gland polyps (OR 2.29, 95% CI: 1.29–4.06) (1,16). Patients with FAP, on the other hand, are much more likely to have duodenal adenomas (up to 90% of patients with FAP will have a duodenal adenoma at some point in their life, and these account for approximately 60% of all duodenal adenomas encountered during EGD). Additionally, FAP-associated duodenal adenomas tend to be multiple, are more likely to involve the ampulla, and arise in patients at a younger age (30–40 years) than sporadic adenomas (5). The Spigelman classification system helps determine the degree of duodenal adenoma burden and the underlying malignancy risk for assessment of endoscopic versus surgical resection and guides the frequency of endoscopic surveillance; a Spigelman score and classification should be documented when assessing duodenal adenomas in a patient with known FAP (17,18).
While there is a clearly a connection between duodenal and colonic adenomas in patients with FAP, the increased risk of finding colonic adenomas in patients with duodenal adenomas extends beyond the presence of FAP alone. For example, in a meta-analysis of 537 patients with SNADAs who also underwent colonoscopy, the risk of having colonic adenomas was significantly increased: for advanced colonic adenoma (OR 4.30, 95% CI: 3.24–5.70) and for colorectal cancer (OR 3.13, 95% CI: 1.38–7.12) (1,19). Therefore, all patients who are found to have a duodenal adenoma should undergo a colonoscopy if they have not had one previously (1).
A study (18) of 43 biopsy-proven SNADAs with only low-grade dysplasia followed longitudinally (mean 27.7 months) without resection showed no histopathological change in 79.1%, progression to high-grade dysplasia in 16.2%, and progression to invasive carcinoma in 4.7%. Lesions containing high-grade dysplasia at the time of initial biopsy and lesions ≥20 mm in size were significantly associated with increased risk of carcinoma development.
Brunner gland polyps
Brunner glands produce a protective alkaline mucous in the duodenum, and occasionally these can proliferate and grow large, mimicking a duodenal lesion. When this occurs, these lesions are referred to as Brunner gland hyperplasia, though the terms “Brunner gland hamartoma” or even “Brunner gland adenoma” are sometimes used. These are commonly encountered in the duodenal bulb and account for approximately 11% of benign duodenal lesions. Although these are not thought to have malignant potential, resection may be indicated if these lesions are symptomatic from bleeding or large size (2,11,20).
Ectopic gastric tissue
Ectopic gastric tissue presenting as gastric foveolar metaplasia can be found in the duodenal bulb and is typically considered a benign reactive process (11). Similarly, pancreatic heterotopia is an ectopic implant of pancreatic tissue which may appear as nondescript nodular tissue or may have a central umbilication and is considered benign (11).
Hamartomas
Hamartomatous polyps are a heterogeneous mixture of stromal and epithelial elements, often presenting as inflammatory polypoid lesions in the duodenum. Solitary Peutz-Jegher polyps are rare hamartomas found in patients without an underlying diagnosis of Peutz-Jegher syndrome (PJS), and when identified, one should consider a work-up for underlying PJS. PJS polyposis, as well as other conditions including juvenile polyposis, Cowden syndrome, and Cronkhite-Canada syndrome, often result in multiple duodenal hamartomatous lesions. These have a risk of malignant transformation, so endoscopic resection of most hamartomatous lesions is generally recommended (2,11).
Subepithelial lesions (SELs)
SELs arise from a layer deep to the mucosa (submucosa, muscularis propria, or serosa) and endoscopically present as extrinsic compression into the duodenum with relatively normal appearing overlying mucosa. Just as with mucosal-based lesions, SELs may be benign, pre-malignant, or malignant, and the majority are asymptomatic.
Lipomas
Lipomas are generally considered benign lesions, though differentiating them from liposarcomas may be challenging. Lipomas typically appear as round, soft lesions. A “positive pillow sign” is when a device (such as a closed biopsy forcep) is pressed into the lesion resulting in a central indentation, and this is indicative of (but not pathopneumonic for) a lipoma. On endoscopic ultrasound (EUS), lipomas appear as homogenous, hyperechoic growths arising from the second or third layer. Bite-on-bite biopsies, also known as submucosal tunneling, which is a technique to access the subepithelial space of a lesion by taking multiple consecutive samples with biopsy forceps in the same location on a lesion, has been used for these (to see extrusion of yellow-colored adipose tissue from the tunnel), though this technique is not recommended for duodenal SELs. Fine needle aspiration/biopsy (FNA/FNB) is generally not required for diagnosis, and because these are not pre-malignant and the majority are asymptomatic, removal is unnecessary (2,21,22).
Gastrointestinal stromal tumors (GISTs)
GISTs arise from the interstitial cells of Cajal, mostly in the muscularis propria. These are much more commonly encountered in the stomach, but duodenal GISTs have a higher likelihood of being symptomatic. Endoscopically, these lesions appear similar to lipomas but do not have positive “pillow sign”. On EUS, they are typically hypoechoic. Diagnosis is most-often made via EUS-directed FNA/FNB. Given that these usually arise from the deep muscle layer, endoscopic removal in the duodenum is very challenging or not possible. Resection should be considered for lesions >20 mm in size, and surgery is more commonly recommended (21,22).
Neuroendocrine tumors
Intestinal neuroendocrine tumors (carcinoids) are relatively rare lesions with less than 5% arising in the duodenum. EUS allows for improved assessment in terms of size, layer of origin, and sampling with FNA/FNB for tissue confirmation. Endoscopic removal of these lesions is typically limited to lesions <20 mm in size arising in the submucosa and most-often require advanced endoscopic resection techniques for removal (using band-assisted EMR, endoscopic full thickness resection device, or ESD) and should not be performed in routine clinical practice. Endoscopic removal of lesions arising in the deep muscle layer is possible, though this comes with the understanding that the resection will be full-thickness, resulting in a perforation requiring endoscopic closure. Multifocal lesions or lesions >20 mm in size should be managed with surgical resection. Often, duodenal carcinoids may be multifocal, so consideration should be given to a thorough EUS exam and cross-sectional imaging studies to evaluate for the presence of other carcinoids within the duodenum as well as for evaluation of malignant nodal disease (2,18).
Endoscopic considerations and assessment
Positioning a duodenal lesion for optimal endoscopic assessment and potential resection is particularly challenging owing to the unique features of the duodenum discussed previously. There are several tools an endoscopist may utilize to improve lesion exposure and visualization. The two most commonly used devices include a clear distal attachment cap and switching to a side-viewing duodenoscope, particularly for lesions near the major papilla. Prior to any duodenal resection, the location of the major and minor papilla in relation to the lesion should be described and photo-documented (1,2,23,24). Pediatric colonoscopes or double channel therapeutic gastroscopes can also be helpful. On a standard gastroscope, the device comes out of the working channel at the seven o’clock position; on a pediatric colonoscope, however, the device comes out at the five o’clock position. This small change can make a big impact and can be particularly helpful for lesions on the posterior/lateral wall, especially in the duodenal bulb or duodenal sweep (2). Additionally, a pediatric colonoscope can be helpful for lesions in the third or fourth portion of the duodenum. A double channel therapeutic gastroscope allows for either working channel to be used for optimal positioning.
Duodenal lesion assessment has many similarities to that in the colorectum. It is important to assess size, morphology (typically using the Paris Classification for standardization of nomenclature), surface pattern, tenting of surrounding mucosa (which can indicate a submucosal invasive component), and determine if the lesion is mucosal-based or is a SEL (2,18). Many of the endoscopes in use today enable high-definition visualization, and although not available on most standard gastroscopes, the use of optical zoom allows for improved visualization of the vascular and pit patterns (24). Virtual chromoendoscopy platforms, like dye-based or NBI, improve duodenal lesion assessment and detection rates (2,24-26). EUS can provide critically important information about SELs but is not typically required for mucosal-based lesions, though consideration for EUS in non-ampullary duodenal adenomas with high-risk features should be given to assess for evidence of an invasive component prior to attempted resection (Figure 7) (8).

Endoscopic removal of duodenal lesions
Prior to starting a resection on any gastrointestinal neoplasia, particularly in the duodenum, the endoscopist should be confident the lesion can be resected in its entirety, as incomplete resection will result in underlying fibrosis which greatly increases the difficulty of subsequent resection attempts and increases the risk of incomplete resection, procedural complications, and the need for surgery (7,27).
Cold biopsy forcep polypectomy
While guidelines now recommend against cold biopsy forcep polypectomy for diminutive colorectal polyps due to higher rates of recurrence compared to cold snare polypectomy (CSP) (28), high-quality head-to-head trials in the duodenum are lacking. Snare polypectomy in the duodenum may be more challenging compared to the colon given the reduced working space and limited scope mobility, leading to difficulty with optimal snare positioning. Therefore, cold biopsy forcep polypectomy in the duodenum may be more appealing to some endoscopists. A multicenter prospective study (29) assessed the safety and efficacy of cold biopsy forcep polypectomy in 39 non-ampullary duodenal adenomas measuring ≤6 mm (range, 3–6 mm; median 5 mm) and showed complete resection without evidence of recurrence at repeat endoscopic assessment at one year in 97.4% (four lesions had recurrence at first follow-up endoscopy at one month, three of which were successfully treated with repeat cold biopsy forcep polypectomy with no recurrence at one year). No adverse events were reported. Despite these findings, the European Society for Gastrointestinal Endoscopy (ESGE) still recommends CSP for lesions <6 mm in size (1).
CSP
CSP is generally favored for small (<10 mm) duodenal mucosal lesions given its safety profile and relative efficacy (30). One retrospective study (31) assessed the efficacy of CSP for 46 sporadic non-ampullary duodenal epithelial lesions (mean size 4.2 mm) and showed an en bloc resection rate of 97.8%, with only one lesion having recurrence at the time of follow-up endoscopy at one year, and there were no perforations or delayed bleeding. The avoidance of electrocautery reduces the risk of perforation, post-polypectomy syndrome, and delayed bleeding, all of which have been shown to be higher in electrocautery-enhanced snare polypectomy in the duodenum compared to the colon (1,27,30). One retrospective cohort study (32) assessed the safety and efficacy of CSP (n=41) to electrocautery-enhanced snare polypectomy (n=69) for non-ampullary duodenal polyps ≤20 mm and found CSP had reduced rates of delayed adverse events (0% vs. 8.7%, P=0.04) with no differences in recurrence rates.
Endoscopic mucosal resection (EMR)
Duodenal EMR has many similarities to colorectal EMR in that both involve placing an injector needle into the submucosal space below the target lesion followed by injection of a solution (such as normal saline or hetastarch with or without methylene blue or indigo carmine, or a wide array of commercially available lifting solutions) followed by snare resection, typically using electrosurgical energy. Additionally, the goal of submucosal injection in both the duodenum and in the colorectum is to raise the polyp off of the muscle layer allowing for improved tissue grasping by the snare and to ideally reduce the risk of deep tissue injury which can result in delayed perforation or bleeding. Using an electrocautery-enhanced snare may help reduce the risk of immediate bleeding and increase rates of en bloc resection. However, the risks of using electrocautery in the duodenum are acute and delayed perforation (which can occur in up to 4% of patients) as well as delayed bleeding (which has been reported to be as high as 29%), all of which are higher than in the colorectum (33-35). Unlike in colorectal EMR where collection of resected tissue is typically the final step of the procedure, resected duodenal tissue is at risk of migrating distally immediately after resection, so the endoscopist should work to collect the resected tissue quickly. Use of glucagon, delivered intravenously at 0.25–0.5 mg doses (typically limited at 1 mg total), can help to reduce duodenal contractions to temporarily reduce the risk of downstream loss of resected specimens.
A 2016 meta-analysis (36) of 14 studies including 485 duodenal polyps (90% were adenomas) in 440 patients with mean size ranging from 13 mm to 35 mm showed an en bloc resection rate of 45%, and 29% required adjunctive ablative therapy for complete resection, with an overall 90% complete resection rate on initial endoscopy. Adverse events included a pooled bleeding rate after EMR of 5% (with risk of bleeding correlating to lesion size) and a 1% perforation rate. After a follow-up time of 6–72 months, there was a 15% recurrence rate (the vast majority of which were able to be successfully treated at subsequent endoscopy).
A single-center study (37) assessed the safety of EMR in 167 non-ampullary duodenal adenomas (mean size 25 mm) and found delayed bleeding in 17.4%, immediate perforation in 2.4%, and delayed perforation in 2.4%. A logistic regression analysis demonstrated that size was the only risk factor for adverse events (OR 2.81, 95% CI: 1.27–6.47, P=0.012). Although recurrence rates following piecemeal EMR vary significantly between studies (0–42.5%), most cases of recurrence are small and can be easily and safely managed endoscopically with low rates or bleeding or perforation (37-41).
Cold piecemeal EMR
While en bloc resection is preferable to piecemeal resection from the standpoints of improved histopathological assessment and reduced recurrence rates, this comes with increased risks of adverse events, particularly bleeding and perforation. As a result, in the past few years there has been an increased interest in cold piecemeal snare resection. This technique comes in various forms, but one of the most commonly applied approaches is a standard submucosal lift (often with a very dilute epinephrine, at 1:100,000 to 1:200,000 concentrations, to reduce the risk of immediate bleeding during the resection which can make visualization challenging) followed by piecemeal cold snare resection (35,42-44). A retrospective single-center study of 43 non-ampullary duodenal polyps ≥10 mm (mean 26.5 mm; range, 10 to 70 mm) removed with piecemeal cold snare EMR showed residual or recurrent adenoma (RRA) in 46% (higher correlation with larger polyps) and ultimate polyp eradication in 89% of patients over the course of a median of two endoscopies. There were no perforations and only one case of post-procedural bleeding (2.3%) (44). To date, there are no prospective head-to-head trials of conventional EMR versus cold piecemeal EMR for non-ampullary duodenal lesions.
Thermal ablation of defect margins
Thermal ablation of the defect margins following EMR of duodenal lesions has been shown to reduce the rates or RRA. One single-center observational study (45) assessed the impact of applying thermal therapy (in the form of low-voltage coagulation using the tip of the snare) to 2–3 mm of the normal mucosa on the rim of the defect margin after EMR (EMR-T) in 54 non-ampullary duodenal adenomas compared to a historical control of 125 similar duodenal adenomas that underwent EMR without thermal ablation (median polyp size 30 mm in both groups). RRA was only seen in 2.3% in the EMR-T group versus 17.6% of the conventional EMR group (P=0.01) without any increase in adverse events.
Underwater EMR (U-EMR)
U-EMR is a technique in which there is no submucosal lifting performed prior to resection; rather, the air/CO2 is suctioned from that section of duodenum and sterile water (or saline) is used to minimally fill the duodenum and provide visualization. The U-EMR technique results in less tension on the walls of the duodenum allowing the mucosa to float away from the deep muscle layer for easier grasping by using the snare and reduced risk of muscle injury or perforation. U-EMR has been shown to increase rates of en bloc resection for duodenal polyps (1,42,46-49). A Japanese retrospective study compared 104 non-ampullary duodenal polyps <20 mm in size removed with U-EMR to a cohort of 240 similar polyps removed with conventional EMR and showed U-EMR had significantly higher rates of technical success (87% vs. 70%, P<0.01), en bloc resection (96% vs. 87%, P<0.01), and R0 resection (80% vs. 67%, P=0.05) without any differences in adverse events (46). A meta-analysis of 8 studies of U-EMR on 258 duodenal lesions (mean size 19.4 mm; range, 6–150 mm) showed a pooled clinical success rate of 89.9% with en bloc removal in 84.6% and a pooled adverse event rate of 6.9% (7 cases of delayed bleeding of which only one required endoscopic therapy, and no cases of perforation) (50). In a retrospective analysis of 44 consecutive patients with 46 sporadic non-ampullary duodenal epithelial lesions (median size 8 mm; range, 2–20 mm) in which 18 were removed with conventional EMR and 28 removed with U-EMR, the U-EMR group had higher rates of en bloc resection (96.4% vs. 72.2%, P<0.05) with reduced resection time (4 vs. 9.5 minutes, P<0.05), and there were no statistically significant differences in rates of recurrence (0% vs. 11.1%, P=0.148) and no adverse events in either group (51).
Cap-assisted EMR (EMR-C)
EMR-C involves suctioning a target lesion into a specialized distal attachment device that is preloaded with a snare followed by hot snare resection. This technique requires submucosal lifting as the action of suctioning the mucosa into the cap increases the risk of inadvertent full-thickness resection by the snare (38,52,53). In a single-center retrospective study (53) of 49 SNADAs treated with EMR-C, 10 lesions were resected en bloc and 39 were resected piecemeal; 90.5% of lesions had no recurrence on the initial follow-up endoscopy, and all areas of recurrence were ultimately successfully managed endoscopically (median follow-up of 17 months). Intra-procedural bleeding occurred in 10.2%, delayed bleeding in 5.1%, and perforation in 1.7%.
Full-thickness resection
Endoscopic full-thickness resection (EFTR) involves the use a specialized distal attachment device that allows for suction of a lesion into the clear cap loaded with an over-the-scope clip (OTSC) which has a separate tissue grasper and an integrated snare, allowing for full-thickness resection without extra-luminal exposure (54). While this technique may be helpful in instances of submucosal fibrosis and even for submucosal lesions, it has significant limitations in the duodenum. The device is bulky, which makes passing it through the posterior oropharynx and navigating to the desired location within the duodenum difficult, and the lesion size is limited by what will fit into the distal attachment cap of the device (generally <2 cm). Moreover, the target lesion needs to be at least 2 cm away from the major and minor papilla to avoid occluding the biliary or pancreatic ducts (1,54). Additionally, the data for use in the duodenum is somewhat limited. In a German study (55) of 20 patients with a total of 20 duodenal lesions, the authors assessed the safety and efficacy of EFTR. All patients underwent a 20 mm balloon dilation of upper esophageal sphincter and the pylorus prior to attempting passage of the EFTR device. Technical success was achieved in 17/20 (85%); the device was able to be advanced to the desired lesion in 19/20 (95%), and minor bleeding was seen on post-procedural day one in 3/19 (15.8%) without any cases of major bleeding or perforation. In general, the data on duodenal EFTR is quite limited, and its use should only be considered in expert centers.
Band ligation-assisted EMR (L-EMR)
L-EMR has been shown to be helpful at endoscopically removing small (<20 mm) SELs in the duodenum but should not generally be employed in duodenal adenoma treatment (56,57). Endoscopic management of duodenal SELs remains controversial, and one must first understand their layer of origin prior to resection attempts. For example, if a lesion originates in the muscularis propria, L-EMR may be incomplete or full-thickness resulting in a perforation requiring complex endoscopic closure. Although this technique is occasionally applied at specialized centers, L-EMR is more commonly utilized in lesions originating in the submucosa. In this technique, bands come pre-loaded onto the outside a specialized clear distal attachment cap, and the lesion of interest is suctioned into the cap with or without submucosal lifting and a band is deployed around the base of the lesion followed by electrocautery-enhanced snare resection. The “pseudo-polyp” created by the band may be resected above or below the band—resection below the band will result in a larger and deeper resection. A major limitation to L-EMR usage is the concern for increased risk of perforation. A study (57) of 15 patients with duodenal carcinoids (mean size 6.6 mm) originating in the submucosal layer compared L-EMR (7 cases) to conventional EMR (8 cases) and showed no differences in rates of en bloc resection (100% vs. 87.5%, P=0.333), perforation (14.3% vs. 0%, P=0.268), or delayed bleeding (0% vs. 12.5%, P=0.333).
Endoscopic submucosal dissection (ESD)
ESD offers the opportunity for higher rates of en bloc resection and reduced recurrence rate compared to EMR (41,58). However, ESD in the duodenum is exceedingly challenging and risky due to the unique characteristics of the duodenum, and this approach should only be performed in high-volume centers with expertise in this procedure (23,30,38,49,59). In fact, the ESGE recommends against the routine usage of ESD for duodenal lesions (and favors standard polypectomy, EMR, or piecemeal EMR) due to the increased risks of bleeding and perforation associated with duodenal ESD (38). Similarly, the American Gastroenterological Association’s Clinical Practice Update on ESD in the United States also recommends that endoscopists refrain from duodenal ESD until after they have extensive experience with ESD elsewhere in the gastrointestinal tract due to the high rates of adverse events (60). Rates of intra-procedural and delayed perforation are significantly higher with ESD than with EMR, with rates with ESD upwards of 40% in some studies (1,38,49,61,62). Similarly, rates of delayed bleeding with duodenal ESD have been reported as high as 22% (41). Technological advances in duodenal ESD include new approaches, like the “pocket” technique, and new devices for resection, coagulation, traction, and closure, all of which may reduce the risk of intra- and post-procedural adverse events. Despite this, the risks of ESD remain high. Delayed perforation may also occur after successful ESD during which there was no breach of the muscularis propria. These factors limit the utility of ESD in the duodenum (41). Lastly, ESD for duodenal adenomas may not be necessary, particularly with newer resection methods (like U-EMR) and post-resection ablative techniques (EMR-T) to reduce the risk of recurrence. A study (63) of 142 patients with sporadic non-ampullary duodenal epithelial tumors treated with EMR or ESD were put into 28 propensity-matched pairs, and the EMR group had significantly reduced procedural time than the ESD group (6 versus 87.5 minutes, P<0.001), reduced hospital stay (8 vs. 11 days, P=0.006), and no differences in rates of en bloc resection (82.1% vs. 92.9%, P=0.42), complete resection (71.4% vs. 89.3%, P=0.18), or adverse events (3.6% vs. 17.9%, P=0.19).
Management after lesion resection
There are few data on prophylactic measures to reduce the risk of complications following duodenal EMR or ESD. For larger lesions (>33% circumferential), the care ranges from post-procedural discharge from endoscopy to hospital admission for monitoring. Similarly, some studies do not mention any post-procedural usage of proton pump inhibitors (PPIs), while others placed patients on twice daily PPI for a period of 2-8 weeks (64). We suggest a two month course of PPI therapy to reduce the risk of acid damage to the de-epithelialized duodenum, even when successful mucosal closure is achieved. The timing of restarting antiplatelet and/or anticoagulation after resection also varies significantly, ranging from not mentioned to restarting one week after the procedure; these decisions must be made on a patient-by-patient basis taking into consideration the clinical need for antiplatelet and/or anticoagulation medications.
The use of through-the-scope clips (TTSCs) after duodenal resection reduces the risk of delayed bleeding. In one study (65) of duodenal EMR on 37 non-ampullary lesions (mean size 19 mm; range 4–50 mm) which had a 97% rate of complete resection on the index endoscopy, there were no cases of post-procedural bleeding following resection of lesions closed with TTSCs whereas 22% of the lesions without closure had bleeding. Similarly, in a study (66) of 26 sporadic non-ampullary duodenal lesions with high-grade dysplasia or intramucosal adenocarcinoma resected with EMR, the usage of TTSCs significantly reduced the rate of delayed bleeding compared to no TTSC usage (0% versus 42.9%, P=0.013).
OTSCs have also been shown to reduce the risk of delayed bleeding. A study (67) of 50 consecutive patients who underwent ESD for sporadic non-ampullary duodenal lesions showed successful closure in 47/50 (94%) of cases, and there were 3 patients (6.3%) with delayed bleeding (all of which were able to be controlled endoscopically); similarly, there was only one case of delayed perforation, and this was in a patient who did not have successful closure. In a study (68) of 249 sporadic non-ampullary duodenal epithelial lesions in 235 consecutive patients, the first 114 were treated with OTSC closure while the remaining 135 were treated with OTSC followed by additional TTSC placed to cover the inverted submucosa after OTSC placement. The group with OTSC + TTSC showed a lower rate of delayed bleeding than the OTSC alone group (1.5% vs. 11.4%, P=0.04) which was supported by a propensity score-matching analysis showing that TTSC added to OTSC significantly reduced the risk of delayed bleeding (P=0.003). OTSCs also likely reduce the risk of delayed perforation following resection of large duodenal lesions. The efficacy of various closure devices to prevent delayed perforation is not as well-studied. Additionally, the efficacy of the through-the-scope helical tack-and-suture device is limited (69).
Post-procedural management plans should be individualized based on a patient’s pre-existing medical comorbidities, usage of antiplatelet/anticoagulation, and details of the procedure itself, including the size and location of the lesion, the resection technique utilized, and any complications (like bleeding, perforation, muscle exposure) that occurring during the procedure. Closure (in the form of TTSC, OTSC, or suturing) is generally recommended following EMR or other advanced resection techniques in the duodenum. Again, there is no clear guidance on the post-procedural usage of PPIs, but a limited course, as might be done to treat a duodenal ulcer, is typically recommended following EMR (1,23,69,70).
The timing of initial and subsequent endoscopic surveillance examinations following duodenal EMR is unclear, but all patients who have undergone duodenal adenoma resection should be considered for endoscopic surveillance for recurrence (23). The ESGE recommends the index reassessment be performed at 3 months and again one year later (1). We suggest waiting 3-6 months after the initial resection to allow for mucosal healing prior to surveillance endoscopy. The recommendations for interval surveillance beyond 1 year are even less clear, and this is an area in much need of future research.
Future endeavors and unanswered questions
Despite the bourgeoning literature in the field of endoscopic resection of non-ampullary duodenal polyps, there are many unanswered questions. It remains unclear which specific endoscopic technique and approach to duodenal polyp resection is the safest and most efficacious. Many studies are single-center retrospective studies, and few studies directly compare one technique to another. Additionally, there is little data on optimal endoscopic closure techniques following resection, and minimal work has been done to assess the safety and efficacy of catheter-based through-the-scope hemostatic agent applications following resection. Finally, more work is needed to improve the understanding of optimal time intervals of endoscopic surveillance following resection.
Conclusions
The unique characteristics of the duodenum increase the risk and complexity of polyp resection compared to elsewhere in the gastrointestinal tract. Although most non-ampullary duodenal epithelial lesions can be effectively removed with standard polypectomy or EMR techniques, lesions requiring advanced maneuvers should only be performed at high-volume centers, and ESD should only be done by experts in this field. Even when duodenal mucosal neoplasia is successfully resected endoscopically, delayed bleeding and delayed perforation can potentially occur, and closure techniques should be considered especially following resection of large lesions. Follow-up is essential to ensure successful long-term endoscopic eradication of neoplasia, though refinement of optimal endoscopic surveillance intervals are needed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Terry L. Jue) for the series “A U. S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” published in Annals of Laparoscopic and Endoscopic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ales.amegroups.com/article/view/10.21037/ales-23-34/rc
Peer Review File: Available at https://ales.amegroups.com/article/view/10.21037/ales-23-34/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ales.amegroups.com/article/view/10.21037/ales-23-34/coif). The series “A U. S. Perspective on Endoscopic Resection of Neoplastic Lesions of the Gastrointestinal Tract” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All images included in this review were obtained during the routine clinical care of the patient [performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013)] and were not performed for the purposes of this review article; additionally, written informed consent was obtained from the patients to perform the procedures. The images contain no patient-specific information or potential identifiers, and, as such, no informed consent for their usage is necessary.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vanbiervliet G, Moss A, Arvanitakis M, et al. Endoscopic management of superficial nonampullary duodenal tumors: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021;53:522-34. [Crossref] [PubMed]
- Gaspar JP, Stelow EB, Wang AY. Approach to the endoscopic resection of duodenal lesions. World J Gastroenterol 2016;22:600-17. [Crossref] [PubMed]
- Nakayama A, Kato M, Masunaga T, et al. Differential diagnosis of superficial duodenal epithelial tumor and non-neoplastic lesion in duodenum by magnified endoscopic examination with image-enhanced endoscopy. J Gastroenterol 2022;57:164-73. [Crossref] [PubMed]
- Lim CH, Cho YS. Nonampullary duodenal adenoma: Current understanding of its diagnosis, pathogenesis, and clinical management. World J Gastroenterol 2016;22:853-61. [Crossref] [PubMed]
- Udd M, Lindström O, Tenca A, et al. Endoscopic therapy of sporadic non-ampullary duodenal adenomas, single centre retrospective analysis. Scand J Gastroenterol 2023;58:208-15. [Crossref] [PubMed]
- Woo SM, Real MJ, Will BM, et al. Clinical outcomes: endoscopic resection of duodenal ampullary lesions. Transl Gastroenterol Hepatol 2023;8:15. [Crossref] [PubMed]
- Bourke MJ. Endoscopic resection in the duodenum: current limitations and future directions. Endoscopy 2013;45:127-32. [Crossref] [PubMed]
- Pavlovic-Markovic A, Dragasevic S, Krstic M, et al. Assessment of Duodenal Adenomas and Strategies for Curative Therapy. Dig Dis 2019;37:374-80. [Crossref] [PubMed]
- Buerlein RCD, Wang AY. The key to reducing residual or recurrent adenoma after duodenal EMR is remembering to spice up the rim. Gastrointest Endosc 2021;93:1381-3. [Crossref] [PubMed]
- Niwa A, Kuwano S, Tomita H, et al. The different pathogeneses of sporadic adenoma and adenocarcinoma in non-ampullary lesions of the proximal and distal duodenum. Oncotarget 2017;8:41078-90. [Crossref] [PubMed]
- Collins K, Ligato S. Duodenal Epithelial Polyps: A Clinicopathologic Review. Arch Pathol Lab Med 2019;143:370-85. [Crossref] [PubMed]
- Kakushima N, Kanemoto H, Tanaka M, et al. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol 2014;20:12501-8. [Crossref] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3-43. [Crossref] [PubMed]
- Lopez-Ceron M, van den Broek FJ, Mathus-Vliegen EM, et al. The role of high-resolution endoscopy and narrow-band imaging in the evaluation of upper GI neoplasia in familial adenomatous polyposis. Gastrointest Endosc 2013;77:542-50. [Crossref] [PubMed]
- Kakushima N, Yoshida M, Yamaguchi Y, et al. Magnified endoscopy with narrow-band imaging for the differential diagnosis of superficial non-ampullary duodenal epithelial tumors. Scand J Gastroenterol 2019;54:128-34. [Crossref] [PubMed]
- Matsuzaki J, Suzuki H, Shimoda M, et al. Clinical and endoscopic findings to assist the early detection of duodenal adenoma and adenocarcinoma. United European Gastroenterol J 2019;7:250-60. [Crossref] [PubMed]
- Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989;2:783-5. [Crossref] [PubMed]
- Okada K, Fujisaki J, Kasuga A, et al. Sporadic nonampullary duodenal adenoma in the natural history of duodenal cancer: a study of follow-up surveillance. Am J Gastroenterol 2011;106:357-64. [Crossref] [PubMed]
- Genta RM, Hurrell JM, Sonnenberg A. Duodenal adenomas coincide with colorectal neoplasia. Dig Dis Sci 2014;59:2249-54. [Crossref] [PubMed]
- Lee WC, Yang HW, Lee YJ, et al. Brunner's gland hyperplasia: treatment of severe diffuse nodular hyperplasia mimicking a malignancy on pancreatic-duodenal area. J Korean Med Sci 2008;23:540-3. [Crossref] [PubMed]
- Gong EJ, Kim DH. Endoscopic Ultrasonography in the Diagnosis of Gastric Subepithelial Lesions. Clin Endosc 2016;49:425-33. [Crossref] [PubMed]
- Jacobson BC, Bhatt A, Greer KB, et al. ACG Clinical Guideline: Diagnosis and Management of Gastrointestinal Subepithelial Lesions. Am J Gastroenterol 2023;118:46-58. [Crossref] [PubMed]
- ASGE Standards of Practice Committee. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc 2015;82:773-81. [Crossref] [PubMed]
- Dekker E, Boparai KS, Poley JW, et al. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy 2009;41:666-9. [Crossref] [PubMed]
- Hurley JJ, Thomas LE, Walton SJ, et al. The impact of chromoendoscopy for surveillance of the duodenum in patients with MUTYH-associated polyposis and familial adenomatous polyposis. Gastrointest Endosc 2018;88:665-73. [Crossref] [PubMed]
- Hüneburg R, Heling D, Kaczmarek DJ, et al. Dye chromoendoscopy leads to a higher adenoma detection in the duodenum and stomach in patients with familial adenomatous polyposis. Endosc Int Open 2020;8:E1308-14. [Crossref] [PubMed]
- Ma MX, Bourke MJ. Management of duodenal polyps. Best Pract Res Clin Gastroenterol 2017;31:389-99. [Crossref] [PubMed]
- Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic Removal of Colorectal Lesions: Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2020;115:435-64. [Crossref] [PubMed]
- Kanzaki H, Horii J, Takenaka R, et al. Prospective multicenter study of the efficacy and safety of cold forceps polypectomy for ≤ 6-mm non-ampullary duodenal low-grade adenomas. Endosc Int Open 2022;10:E712-8. [Crossref] [PubMed]
- Ochiai Y, Kato M, Kiguchi Y, et al. Current Status and Challenges of Endoscopic Treatments for Duodenal Tumors. Digestion 2019;99:21-6. [Crossref] [PubMed]
- Okimoto K, Maruoka D, Matsumura T, et al. Long-term outcomes of cold snare polypectomy for superficial non-ampullary duodenal epithelial tumors. J Gastroenterol Hepatol 2022;37:75-80. [Crossref] [PubMed]
- Trivedi M, Klapheke R, Youssef F, et al. Comparison of cold snare and hot snare polypectomy for the resection of sporadic nonampullary duodenal adenomas. Gastrointest Endosc 2022;96:657-664.e2. [Crossref] [PubMed]
- Probst A, Freund S, Neuhaus L, et al. Complication risk despite preventive endoscopic measures in patients undergoing endoscopic mucosal resection of large duodenal adenomas. Endoscopy 2020;52:847-55. [Crossref] [PubMed]
- Sohn JW, Jeon SW, Cho CM, et al. Endoscopic resection of duodenal neoplasms: a single-center study. Surg Endosc 2010;24:3195-200. [Crossref] [PubMed]
- Patel V, Cassani L. Cold snare polypectomy in the small bowel: Are we ready to turn down the heat? Gastrointest Endosc 2022;95:1183-5. [Crossref] [PubMed]
- Navaneethan U, Hasan MK, Lourdusamy V, et al. Efficacy and safety of endoscopic mucosal resection of non-ampullary duodenal polyps: a systematic review. Endosc Int Open 2016;4:E699-708. [Crossref] [PubMed]
- Amoyel M, Belle A, Dhooge M, et al. Endoscopic management of non-ampullary duodenal adenomas. Endosc Int Open 2022;10:E96-E108. [Crossref] [PubMed]
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Alexander S, Bourke MJ, Williams SJ, et al. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc 2009;69:66-73. [Crossref] [PubMed]
- Valli PV, Mertens JC, Sonnenberg A, et al. Nonampullary Duodenal Adenomas Rarely Recur after Complete Endoscopic Resection: A Swiss Experience Including a Literature Review. Digestion 2017;96:149-57. [Crossref] [PubMed]
- Akahoshi K, Kubokawa M, Inamura K, et al. Current Challenge: Endoscopic Submucosal Dissection of Superficial Non-ampullary Duodenal Epithelial Tumors. Curr Treat Options Oncol 2020;21:98. [Crossref] [PubMed]
- Draganov PV. Pearls and Pitfalls of Endoscopic Resection ofDuodenal Adenomas. Gastroenterol Hepatol (N Y) 2020;16:149-51. [PubMed]
- Javia SB, Chathadi K. Cold snare piecemeal endoscopic mucosal resection of a very large duodenal adenoma. Endoscopy 2019;51:E217-8. [Crossref] [PubMed]
- Dang DT, Suresh S, Vance RB, et al. Outcomes of cold snare piecemeal EMR for nonampullary small-bowel adenomas larger than 1 cm: a retrospective study. Gastrointest Endosc 2022;95:1176-82. [Crossref] [PubMed]
- Sidhu M, Fritzsche JA, Klein A, et al. Outcomes of thermal ablation of the defect margin after duodenal endoscopic mucosal resection (with videos). Gastrointest Endosc 2021;93:1373-80. [Crossref] [PubMed]
- Kiguchi Y, Kato M, Nakayama A, et al. Feasibility study comparing underwater endoscopic mucosal resection and conventional endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumor < 20 mm. Dig Endosc 2020;32:753-60. [Crossref] [PubMed]
- Yamasaki Y, Uedo N, Takeuchi Y, et al. Underwater endoscopic mucosal resection for superficial nonampullary duodenal adenomas. Endoscopy 2018;50:154-8. [PubMed]
- Shibukawa G, Irisawa A, Sato A, et al. Endoscopic Mucosal Resection Performed Underwater for Nonampullary Duodenal Epithelial Tumor: Evaluation of Feasibility and Safety. Gastroenterol Res Pract 2018;2018:7490961. [Crossref] [PubMed]
- Kato M, Kanai T, Yahagi N. Endoscopic resection of superficial non-ampullary duodenal epithelial tumor. DEN Open 2022;2:e54. [Crossref] [PubMed]
- Bhogal N, Mohan B, Chandan S, et al. Efficacy and safety of underwater endoscopic mucosal resection for superficial non-ampullary duodenal epithelial tumors: a systematic review and meta-analysis. Ann Gastroenterol 2020;33:379-84. [Crossref] [PubMed]
- Furukawa M, Mitoro A, Ozutumi T, et al. Efficacy of Underwater Endoscopic Mucosal Resection for Superficial Non-Ampullary Duodenal Epithelial Tumor. Clin Endosc 2021;54:371-8. [Crossref] [PubMed]
- Conio M, De Ceglie A, Filiberti R, et al. Cap-assisted EMR of large, sporadic, nonampullary duodenal polyps. Gastrointest Endosc 2012;76:1160-9. [Crossref] [PubMed]
- Jamil LH, Kashani A, Peter N, et al. Safety and efficacy of cap-assisted EMR for sporadic nonampullary duodenal adenomas. Gastrointest Endosc 2017;86:666-72. [Crossref] [PubMed]
- Hajifathalian K, Ichkhanian Y, Dawod Q, et al. Full-thickness resection device (FTRD) for treatment of upper gastrointestinal tract lesions: the first international experience. Endosc Int Open 2020;8:E1291-301. [Crossref] [PubMed]
- Bauder M, Schmidt A, Caca K. Endoscopic full-thickness resection of duodenal lesions-a retrospective analysis of 20 FTRD cases. United European Gastroenterol J 2018;6:1015-21. [Crossref] [PubMed]
- Neumann H, Ramesh J, Wilcox CM, et al. Resection of carcinoids in the duodenal bulb using the band ligation technique with the Duette mucosectomy device. Endoscopy 2013;45 Suppl 2 UCTN:E365-6.
- Park SB, Kang DH, Choi CW, et al. Clinical outcomes of ligation-assisted endoscopic resection for duodenal neuroendocrine tumors. Medicine (Baltimore) 2018;97:e0533. [Crossref] [PubMed]
- Nishizawa T, Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: technique and new directions. Curr Opin Gastroenterol 2017;33:315-9. [Crossref] [PubMed]
- Whitfield AM, Bourke MJ. Preventing adverse events after endoscopic resection of duodenal polyps: Size and context matter! Gastrointest Endosc 2021;93:375-7. [Crossref] [PubMed]
- Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol 2019;17:16-25.e1. [Crossref] [PubMed]
- Hoteya S, Furuhata T, Takahito T, et al. Endoscopic Submucosal Dissection and Endoscopic Mucosal Resection for Non-Ampullary Superficial Duodenal Tumor. Digestion 2017;95:36-42. [Crossref] [PubMed]
- Basford PJ, George R, Nixon E, et al. Endoscopic resection of sporadic duodenal adenomas: comparison of endoscopic mucosal resection (EMR) with hybrid endoscopic submucosal dissection (ESD) techniques and the risks of late delayed bleeding. Surg Endosc 2014;28:1594-600. [Crossref] [PubMed]
- Esaki M, Haraguchi K, Akahoshi K, et al. Endoscopic mucosal resection vs endoscopic submucosal dissection for superficial non-ampullary duodenal tumors. World J Gastrointest Oncol 2020;12:918-30. [Crossref] [PubMed]
- Yamamoto H, Miura Y. Duodenal ESD: conquering difficulties. Gastrointest Endosc Clin N Am 2014;24:235-44. [Crossref] [PubMed]
- Lépilliez V, Chemaly M, Ponchon T, et al. Endoscopic resection of sporadic duodenal adenomas: an efficient technique with a substantial risk of delayed bleeding. Endoscopy 2008;40:806-10. [Crossref] [PubMed]
- Maruoka D, Arai M, Kishimoto T, et al. Clinical outcomes of endoscopic resection for nonampullary duodenal high-grade dysplasia and intramucosal carcinoma. Endoscopy 2013;45:138-41. [Crossref] [PubMed]
- Tashima T, Ohata K, Sakai E, et al. Efficacy of an over-the-scope clip for preventing adverse events after duodenal endoscopic submucosal dissection: a prospective interventional study. Endoscopy 2018;50:487-96. [Crossref] [PubMed]
- Ohata K, Nonaka K, Sakai E, et al. Novel technique of endoscopic full-thickness resection for superficial nonampullary duodenal neoplasms to avoid intraperitoneal tumor dissemination. Endosc Int Open 2016;4:E784-7. [Crossref] [PubMed]
- Fujihara S, Mori H, Kobara H, et al. Management of a large mucosal defect after duodenal endoscopic resection. World J Gastroenterol 2016;22:6595-609. [Crossref] [PubMed]
- Tsutsumi K, Kato M, Kakushima N, et al. Efficacy of endoscopic preventive procedures to reduce delayed adverse events after endoscopic resection of superficial nonampullary duodenal epithelial tumors: a meta-analysis of observational comparative trials. Gastrointest Endosc 2021;93:367-374.e3. [Crossref] [PubMed]
Cite this article as: Buerlein RCD, Wang AY. Endoscopic resection of non-papillary neoplastic lesions of the duodenum: a narrative review of clinical application and techniques. Ann Laparosc Endosc Surg 2024;9:22.


