Advanced technique of reduced-port laparoscopic total gastrectomy for gastric cancer
Introduction
Nowadays, laparoscopic procedures are performed in many types of gastrointestinal surgery, and laparoscopic surgery has even become a common treatment for gastrointestinal malignant tumors. Furthermore, the advance of laparoscopic instruments brought the next laparoscopic procedures such as single-port laparoscopic surgery (SPLS) or reduced-port surgery (RPS). RPS which uses SPLS technique, has recently become widespread in various types of surgery, such as cholecystectomy, splenectomy, colectomy, and gynecological surgery (1-14). SPLS or RPS is expected to be part of the next generation of surgery, for which the concept is to target fewer or smaller wounds than are targeted by conventional laparoscopic surgery. And the cosmetic merit of RPS is widely accepted (3,4,6,9,11,13). RPS has been increasingly performed even in advanced laparoscopic surgery such as reduced-port laparoscopic gastrectomy (RPG). However, there have been some retrospective studies of small numbers of patients undergoing RPG procedures, including reports of our initial experiences (15-21). Gastrectomy for gastric cancer involves systematic lymph node dissection, and dissection of many vessels, which complicates the process. However, application of SPLS or RPS can reduce the number of ports required for laparoscopic gastrectomy. We started performing RPG through an umbilical multichannel port and an additional port [called dual-port laparoscopic gastrectomy (DP-LG)] for gastric cancer in December 2009, and accumulated experience of 100 cases. In the DP-LG, 79 patients underwent DP-laparoscopic distal gastrectomy (DP-LDG) and 21 underwent DP-laparoscopic total gastrectomy (DP-LTG).
In this report, we explained our methods and devices of DP-LTG, which is the most difficult procedure in the field of PRGs.
Patient selection and workup
The indication for DP-LG was preoperative clinical stage I and II gastric cancer, without a history of upper abdominal surgery. All DP-LTG procedures were performed from December 2010 by a single surgeon who had performed >300 five ports laparoscopic gastrectomy (5P-LG) procedures. Informed consent was obtained by the surgeon from all patients. The information provided to all patients about DP-LG and 5P-LG included notification that the surgeon had adequate experience of laparoscopic gastrectomy and RPS. Patients were also informed that RPS was not an established procedure for gastric cancer and there was the possibility of conversion to 5P-LG.
Procedure
Port setting and devices used in DP-LATG
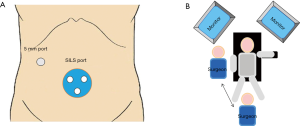
Patients were placed in Fowler’s position with legs abducted. A SILS™ port (Covidien Japan Inc, Tokyo, Japan) with three in-built trocars was inserted into an umbilical incision, while another 5-mm port was inserted in the right flank region (Figure 1A). A 5-mm flexible scope was inserted through the 5-mm trocar at the extreme caudal position of the SILS™ port. The surgeon used two trocars in the SILS™ port to manipulate the greater curvature side of the stomach, or one trocar in the SILS™ port and another in the right flank port to manipulate the other side. The surgeon stood between the patient’s legs in the former situation and on the right side of the patient in the latter (Figure 1B). The assistant used the remaining trocar to provide support. Normal straight graspers and curved-type graspers (Roticulator™ Endo Grasp™; Covidien Japan Inc) were used for grasping the tissue. Activating laparoscopic coagulating shears were used for dissection. D1+ lymph node dissection was performed in patients with cT1 gastric cancer, and D2-No.10 lymph nodes were performed in patients with cT2/3 gastric cancer. The extent of lymph node dissection and cancer stage were classified using the Japanese Classification of Gastric Carcinoma: 3rd English edition and the Japanese Gastric Cancer Treatment Guidelines 2010 published by the Japanese Gastric Cancer Association (22,23).
The steps of the DP-LATG
Surgery of the greater curvature side
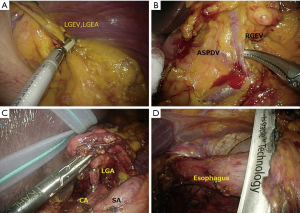
The surgeon stood between the patient’s legs and used the upper two 5 mm trocars in the SILSTM port. The assistant grasped and raised the stomach wall using the grasper inserted through the right flank port. The operator subsequently dissected the omentum as well as the left and right gastroepiploic vessels (Figure 2A,B).
Transection of the duodenum
The surgeon moved to the right side of the patient after manipulating the greater curvature side. The left upper 5 mm trocar of the SILSTM port was replaced with the 12 mm trocar to insert the endolinear stapler used for transection of the duodenum. After performing transection of the duodenum, the 12 mm port was replaced with the 5 mm trocar.
Lesser curvature side and lymph node dissection of the superior side of the pancreas
The surgeon used the right upper or the left upper trocar of the SILSTM port for the LCS and the right flank port for the grasper. The assistant used the remaining trocar of the SILSTM port for retraction. The right gastric artery/vein, lesser omentum, lymph node of the superior side of the pancreas (Nos. 8a, 11p), and left gastric vein/artery were dissected sequentially (Figure 2C).
Dissection of the esophagus and lymph node dissection along the distal splenic artery
The left upper 5 mm trocar of the SILSTM port was replaced with the 12 mm trocar to insert the linear stapler, and the esophagus was dissected. To ease lymph node dissection along the distal splenic artery (No. 11d), this dissection was performed after amputating the esophagus (Figure 2D).
Extraction of the stomach and Y-limb anastomosis
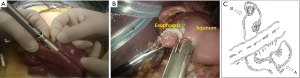
The SILSTM port was removed, and a wound protector was attached to the umbilical incision. The stomach was extracted from the umbilical incision, and a Y-limb anastomosis was created directly (Figure 3A).
Esophagojejunostomy
The SILSTM port was reattached, and esophagojejunostomy was performed laparoscopically by side-to side anastomosis using a linear stapler (Figure 3B,C). The incision used to insert the linear stapler was closed using interrupted sutures. At the end of the operation, a drainage tube was inserted through the port wound in the right flank region.
Post-operative management
The same postoperative care was provided for both DP-LTG and 5P-LTG patients using the same clinical course, with walking and drinking resuming on postoperative day (POD) 1. Rice porridge meals of 30%, 50% and 70% concentrations were resumed on PODs 4, 5 and 6, respectively. Normal rice porridge and normal meals were resumed on PODs 7 and 8, respectively. Patients were discharged from POD 10. The background of the patients in the DP-LTG is shown in Table 1. The operative results of the DP-LTG are shown in Table 2. No patient required an additional port or conversion to open surgery, and no intra- and post-operative complications was seen.
Table 1
| Characteristics | DP-LTG (n=21) |
|---|---|
| Age | 67.1±7.4 |
| Sex | |
| Male | 18 (85.7%) |
| Female | 3 (14.3%) |
| BMI | 23.8±1.9 |
| ASA | |
| 1 | 5 (23.8%) |
| 2 | 16 (76.2%) |
| 3 | 0 (0%) |
| Clinical stage | |
| I | 20 (95.2%) |
| II | 1 (4.8%) |
DP-LTG, dual-port laparoscopic total gastrectomy; BMI, body mass index; ASA, American Society of Anesthesiology classification.
Table 2
| Variables | DP-LTG (n=21) |
|---|---|
| Operation time (min) | 260.0±49.2 |
| Blood loss (mL) | 40.9±44.9 |
| Requiring additional port(s) | |
| + | 0 (0%) |
| − | 21 (100%) |
| Conversion to open surgery | |
| + | 0 (0%) |
| − | 21 (100%) |
| Intraoperative complication | |
| + | 0 (0%) |
| − | 21 (100%) |
| Lymph node dissection | |
| D1 | 0 (0%) |
| D1+ | 20 (95.2%) |
| D2 | 1 (4.8%) |
| Number of harvested lymph nodes | 36.0±10.4 |
| Pathological stage | |
| I | 20 (95.2%) |
| II | 1 (4.8%) |
| III | 0 (0%) |
| IV | 0 (0%) |
| Postoperative morbidity | |
| + | 0 (0%) |
| − | 21 (100%) |
| Postoperative mortality | |
| + | 0 (0%) |
| − | 21 (100%) |
| Times of analgesic requirements | 3.7±2.9 |
| Postoperative hospital stay (day) | 11.7±1.9 |
DP-LTG, dual-port laparoscopic total gastrectomy.
Tips, tricks and pitfalls
On performing RPG, although it has much cosmetic merit, prolongation of the operation time or increasing the morbidity rate should be avoided. Furthermore, achieving a high rate of success is important for its feasibility. Prolongation of operation time is a concern with RPG. In our early experiences of RPG, operation time was significantly longer than that of conventional laparoscopic gastrectomy (16,19,20). This suggests that the operation time for RPG was longer than that of conventional laparoscopic gastrectomy at the outset; however, it improved with experience. We consider two technical reasons for this. First, using a supportive port could have created a wider area for manipulation.
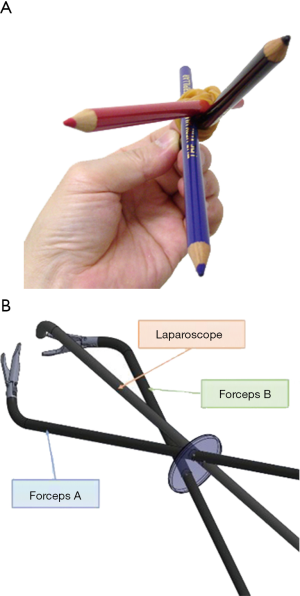
Next, one disadvantage of SPLS and RPS is the lack of free movement of the instruments, resulting in conflict among the forceps, cutting device, and scope. Therefore, the key to smooth performance of RPS for gastric cancer lies in understanding the formation in which there is the highest degree of freedom of movement. When three instruments are passed into a narrow space, only two patterns of shaft formation can be obtained: a formation in which the three instruments rotate clockwise or counterclockwise (rotation formation) (Figure 4A,B), or a formation in which one instrument passes between the other two (cross formation) (Figure 5). A mechanical analysis demonstrated that the rotation formation is ideal, with excellent instrument operability (24). So we used the rotation formation to perform not only in DP-LTG but DP-LDG.
RPG was performed safely and successfully even for total gastrectomy. At present, RPG should be carried out by surgeons with experience in both laparoscopic surgery and PRS, however, it is expected to become an advanced technique in laparoscopic gastrectomy in the future.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ales.2016.11.16). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reavis KM, Hinojosa MW, Smith BR, et al. Single-laparoscopic incision transabdominal surgery sleeve gastrectomy. Obes Surg 2008;18:1492-4. [Crossref] [PubMed]
- Nguyen NT, Hinojosa MW, Smith BR, et al. Single laparoscopic incision transabdominal (SLIT) surgery-adjustable gastric banding: a novel minimally invasive surgical approach. Obes Surg 2008;18:1628-31. [Crossref] [PubMed]
- Gill IS, Canes D, Aron M, et al. Single port transumbilical (E-NOTES) donor nephrectomy. J Urol 2008;180:637-41; discussion 641. [Crossref] [PubMed]
- Teixeira J, McGill K, Binenbaum S, et al. Laparoscopic single-site surgery for placement of an adjustable gastric band: initial experience. Surg Endosc 2009;23:1409-14. [Crossref] [PubMed]
- Fader AN, Escobar PF. Laparoendoscopic single-site surgery (LESS) in gynecologic oncology: technique and initial report. Gynecol Oncol 2009;114:157-61. [Crossref] [PubMed]
- Hernandez JM, Morton CA, Ross S, et al. Laparoendoscopic single site cholecystectomy: the first 100 patients. Am Surg 2009;75:681-5; discussion 685-6. [PubMed]
- Saber AA, Elgamal MH, Itawi EA, et al. Single incision laparoscopic sleeve gastrectomy (SILS): a novel technique. Obes Surg 2008;18:1338-42. [Crossref] [PubMed]
- Goel RK, Kaouk JH. Single port access renal cryoablation (SPARC): a new approach. Eur Urol 2008;53:1204-9. [Crossref] [PubMed]
- Remzi FH, Kirat HT, Kaouk JH, et al. Single-port laparoscopy in colorectal surgery. Colorectal Dis 2008;10:823-6. [Crossref] [PubMed]
- Desai MM, Rao PP, Aron M, et al. Scarless single port transumbilical nephrectomy and pyeloplasty: first clinical report. BJU Int 2008;101:83-8. [Crossref] [PubMed]
- Hong TH, You YK, Lee KH. Transumbilical single-port laparoscopic cholecystectomy: scarless cholecystectomy. Surg Endosc 2009;23:1393-7. [Crossref] [PubMed]
- Langwieler TE, Nimmesgern T, Back M. Single-port access in laparoscopic cholecystectomy. Surg Endosc 2009;23:1138-41. [Crossref] [PubMed]
- Tacchino R, Greco F, Matera D. Single-incision laparoscopic cholecystectomy: surgery without a visible scar. Surg Endosc 2009;23:896-9. [Crossref] [PubMed]
- Barbaros U, Dinççağ A. Single incision laparoscopic splenectomy: the first two cases. J Gastrointest Surg 2009;13:1520-3. [Crossref] [PubMed]
- Omori T, Oyama T, Akamatsu H, et al. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 2011;25:2400-4. [Crossref] [PubMed]
- Kawamura H, Tanioka T, Funakoshi T, et al. Dual-ports laparoscopy-assisted distal gastrectomy compared with conventional laparoscopy-assisted distal gastrectomy. Surg Laparosc Endosc Percutan Tech 2011;21:429-33. [Crossref] [PubMed]
- Kawamura H, Tanioka T, Kuji M, et al. The initial experience of dual port laparoscopy-assisted total gastrectomy as a reduced port surgery for total gastrectomy. Gastric Cancer 2013;16:602-8. [Crossref] [PubMed]
- Kashiwagi H, Kumagai K, Monma E, et al. Dual-port distal gastrectomy for the early gastric cancer. Surg Endosc 2015;29:1321-6. [Crossref] [PubMed]
- Kunisaki C, Ono HA, Oshima T, et al. Relevance of reduced-port laparoscopic distal gastrectomy for gastric cancer: a pilot study. Dig Surg 2012;29:261-8. [Crossref] [PubMed]
- Kunisaki C, Makino H, Kimura J, et al. Application of reduced-port laparoscopic total gastrectomy in gastric cancer preserving the pancreas and spleen. Gastric Cancer 2015;18:868-75. [Crossref] [PubMed]
- Kim SM, Ha MH, Seo JE, et al. Comparison of Reduced Port Totally Laparoscopic Distal Gastrectomy (Duet TLDG) and Conventional Laparoscopic-Assisted Distal Gastrectomy. Ann Surg Oncol 2015;22:2567-72. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Gastric Cancer 2011;14:101-12. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Kawamura H, Ishii C. Mechanical analysis of the formation of forceps and scope for single-port laparoscopic surgery. Surg Laparosc Endosc Percutan Tech 2012;22:e168-75. [Crossref] [PubMed]
Cite this article as: Kawamura H, Yoshida T, Ohno Y, Ichikawa N, Homma S, Taketomi A. Advanced technique of reduced-port laparoscopic total gastrectomy for gastric cancer. Ann Laparosc Endosc Surg 2016;1:41.